How it Works
The Science Behind How Zeolite Works
Science Based Innovation
IMAGINE Zeolite is dedicated to developing science based zeolite solutions that offer safer and economically efficient alternatives to the status quo.
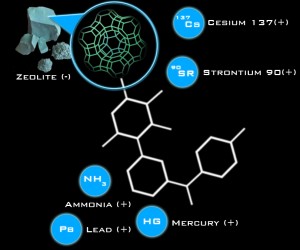
Zeolite have a high CATION ( prounounced “Cat” ‘Ion” a capacity which essentially is an action similar to a magnet attracting iron filings and holding them there.
Zeolites typically prefer larger cations such as heavy metals ( lead, mercury, arsenic, cadmium etc..) ammonia, radionuclides, and a variety of organic molecules.
Click here to see Major Exchangeable Cations
Click here to see Primary Adsorbing Gases
Due to their unique porous crystal structure, Zeolites are capable of absorbing large amounts of water and other substances. This occurs mainly on a molecular level where the holes and channels within the 3-dimensional honeycomb account for this phenomenon. The channel size, specific for each mineral species, controls what can enter the crystal. For this reason, Zeolites are commonly referred to as “molecular sieves”.
Zeolites have a cage-like structure, with pores and channels of specific size running through the crystal. The specific size of the pore lets only smaller sized toxin molecules pass through the pore and into the cage. Larger toxins or substances will not pass through the set pore hole. The toxins outside the pore have a positive charge that is electrically attracted to the pore opening. The negatively charged clinoptilolite cage carries a net negative charge and draws the positively charged toxin to itself trap the positively charged heavy metals and other toxic substances.

